Devatis AG was founded in 2018 in Cham, Switzerland. Behind Devatis AG is EastPharma Ltd, which was established in 2006 with the aim of becoming a major player in the pharmaceutical industry and had been listed on London Stock Exchange (LSE) between 2008 and August 2019.
Currently, the porfolio of Devatis AG consists of hematology, oncology, calcimimetics and antibiotics products and is still growing into other therapeutic areas such as cardiology and anti-fungal.
DEVA Holding, pioneering Turkish pharmaceutical company, was founded in 1958 by group of medical doctors and pharmacists with main scope of business including manufacturing and marketing of high quality pharmaceutical finished products and active ingredients. Company has export operations to more than 60 countries worldwide.
Our Philosophy: We are committed to improving access to quality medicines.
Our Corporate Goal: Continuous growth by expanding our product portfolio to establish a strong brand.
DEVA Holding A.Ş. was founded in 1958 by a group of doctors, pharmacists and veterinarians. Since 1986 DEVA Holding shares have been traded on Istanbul Stock Exchange. Majority shares of DEVA Holding were acquired in 2006 by the funds managed by GEM Global Equities Management S.A., an international fund management company, and EastPharma Ltd. was established to take on the management.
With its main scope of business covering manufacturing and marketing of medicines for human use and raw materials, DEVA Holding also produces veterinary drugs, medical vials. Having a strong presence in the domestic market, DEVA Holding also exports finished products and raw materials to more than 60 countries worldwide.
Growing over 60 years in the Turkish pharmaceutical market, DEVA, with its broad product portfolio in different therapeutic areas, became one of the leading pharmaceutical companies in Turkey. With its three EU-GMP accredited manufacturing facilities, approximately 2900 employees and state-of-the-art R&D centre DEVA targets to become a world-class player. Focusing on developing innovative new forms and products of high added value, DEVA allocated approximately 7 % of its turnover to R&D in 2023.
Deva Holding completed year 2023 successfully closed te year ranking 3rd with 5.2% market share in term of boxes and 6th with 2.9% market share in TL, based on IQVIA data.
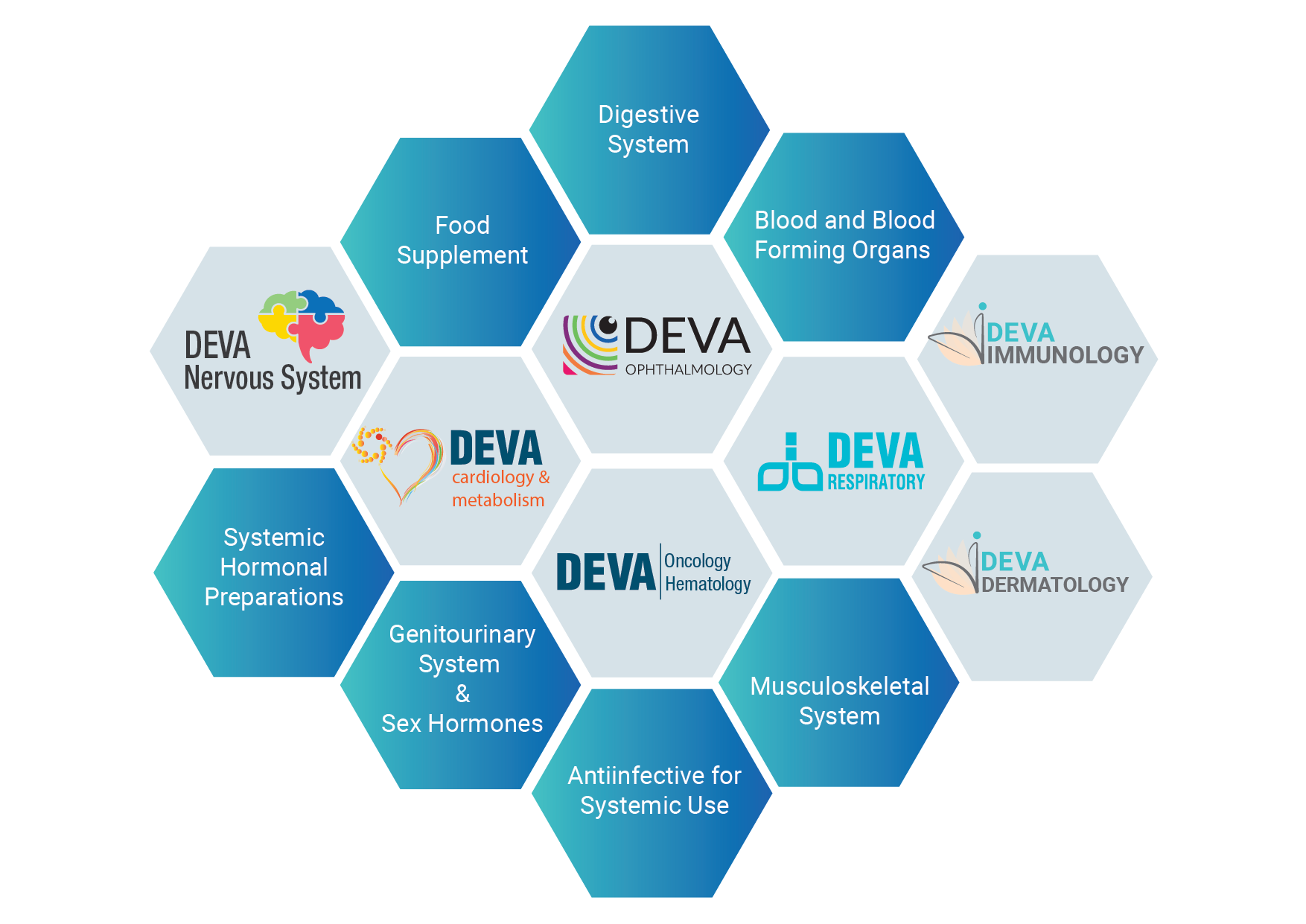
DEVA Holding has more than 650 products in 14 therapeutic areas including oncology, ophthalmology and respiratory system products among others. DEVA Holding has been creating difference by releasing new products into market each year consistently.
DEVA's manufacturing capacity allows production of different pharmaceutical forms at large scales:
Non-sterile
Sterile
The high quality of DEVA production facilities are endorsed by globally recognized certification bodies:
DEVA has three manufacturing facilities which are endorsed by internationally recognized certifications proving the high quality standards of DEVA.
Location: Çerkezkoy/Turkey

Solid Manufacturing Unit
Liquid / Semi Solid Manufacturing Unit
Cephalosporin Manufacturing Unit
Penicilin Manufacturing Unit
Hormone Products Manufacturing Unit (Non sterile)
Hormone Products Manufacturing Unit (Sterile)
Inhaled Products
Sterile Liquid Vial and Soft Gel Capsule Products
Location: Çerkezkoy/Turkey

API Production Units
Human Finished Dosage Forms Production Units
Animal Health Products Production Units
Solids (tablets & powders)
Location: Turkey’s Largest Logistics Center

Turkey's largest pharmaceutical logistics center with 58,000 m2 total land area, which received EU GMP approval, conducts its activities with a capacity of 43,500 pallets.
In 2018, Turkey’s largest pharmaceutical logistics center, started its operations with 32000 pallet capacity and received EU GMP approval
Location: Kartepe/Turkey

Empty& filled ampoule production
Sterile vial & lyophilisation production
SVP production with BFS technology
Eye drop production in sterile PE bottle
Cologne production